Pipeline
Main pipeline products
In the nucleic acid medicine and genomic drug discovery businesses, AnGes is promoting three projects: HGF gene therapy products for Chronic arterial occlusive disease, NF-κB decoy oligonucleotide for Chronic discogenic lumber back pain, and the development of DNA vaccines.
Approval Process
| Project | Area | Partner | Dosage Form | Indication | Basic research | Preclinical study | Clinical trial | Application for Approval | Approval | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | |||||||||
| HGF gene therapy product |
Japan | ー | Injection | Arteriosclerosis obliterans with lower limb ulcer |
in preparation |
||||||
| USA | ー | Injection | Arteriosclerosis obliterans with lower limb ulcer |
P2b Completed |
Preliminary report shows good results |
||||||
| Israel | Kamada | Injection | Chronic arterial occlusive disease with lower limb ulcer |
||||||||
| Turkey | Er-Kim | Injection | Chronic arterial occlusive disease with lower limb ulcer |
||||||||
| NF-κB Decoy Oligonucleotide |
JP | ー | Injection | Chronic discogenic lumber back pain |
On going | ||||||
| DNA Vaccine | Australia | ー | Injection | Hypertension | Completed | ||||||
| DNA Vaccine | USA | ー | Intranasal formulation |
COVID-19 | Completed | ||||||
| Tie2 agonists | USA | Vasomune | Injection | COVID-19 / ARDS | Completed | P2a (on going) |
|||||
※In addition to the above projects, the development pipeline includes drugs for chronic hepatitis B in the exploratory, basic research and pre-clinical stages.
EmendoBio’s Pipeline
| Project | Area | Indication | LEAD OPTIMIZATION | PRE-CLINICAL | IND-ENABLING | PHASE 1-3 |
|---|---|---|---|---|---|---|
| Development of genome editing |
US | Severe Congenital Neutropenia |
||||
| Diseases in hematology, ophthalmology, immuno-oncology, etc. |
||||||
Product for HGF gene therapy
Gene therapy is an approach whereby a specific gene is introduced into the patient’s body and the protein produced by the gene acts as a drug to treat a targeted disease. In 2019, as a world first, AnGes succeeded in commercializing an HGF gene therapy product using circular DNA, plasmid DNA.
In Japan in 1984, a growth factor was found in the liver, the organ with the highest regenerative potential. The factor, which was named hepatocyte growth factor (HGF), was found to play a major role in the formation and regeneration of not only the liver but also several other organs and tissues, including the blood vessels, lymphatic vessels, and nerves. In 1995, the research team led by Professor Ryuichi Morishita and colleagues at Osaka University discovered that HGF has the capacity to generate new vessels, and began the development of an HGF gene therapy product, a therapeutic drug which has the unprecedented ability to generate new vessels for the treatment of ischemic diseases where the blood vessels have become occluded and blood flow has been reduced.
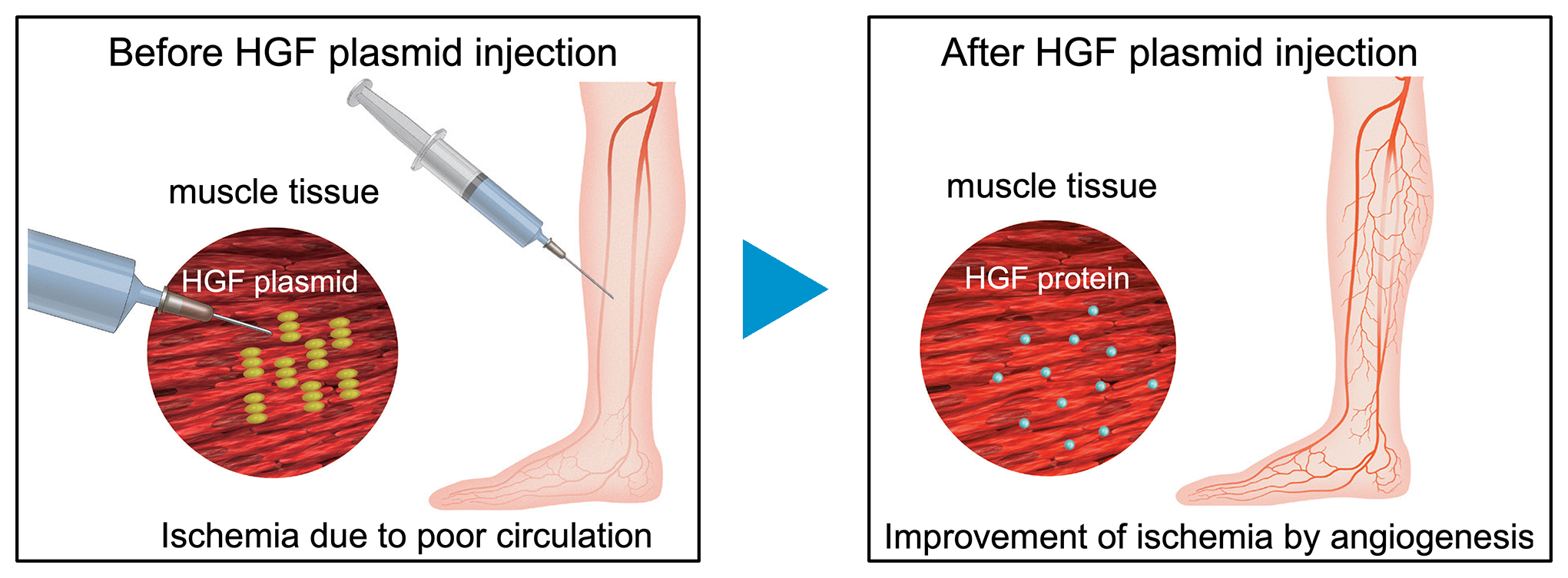
There are several different approaches in gene therapy: development of virus vector and plasmid DNA as a method for gene transfer, and development of genome editing as a repair of disease-causing genes. Plasmid DNA therapy was adopted to reduce the strain on the patient's body and promote angiogenesis. In 2019, as a world first, an HGF gene therapy product using circular DNA, plasmid DNA to improve severe Chronic arterial occlusive disease with lower limb ulcer was released into the market.
productFirst in Japan*
product **First in the world*
using HGFFirst in the world*
product for
generating
new peripheral
blood vesselsFirst in the world*
product for use in
cardiovascular medicineFirst in the world*
* Information as of the time of conditional and time-limited approval for manufacturing and marketing in March 2019.
** Approval criteria of the following three organizations - FDA (Food Drug Administration; US) / EMA (European Medicines Agency; EU) / Ministry of Health, Labour and Welfare (Japan)
NF-κB Decoy Oligo DNA
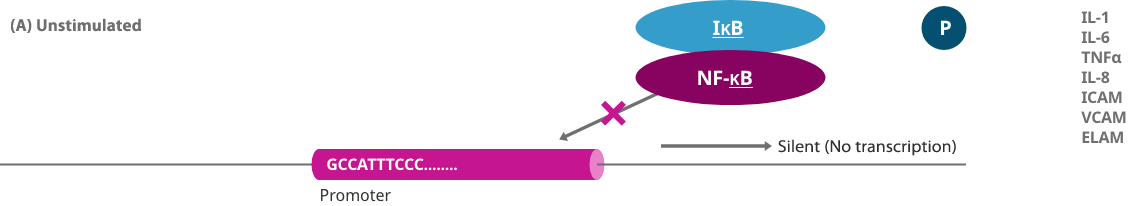
NF-κB, a major transcription factor, initiates induction of inflammatory and immune response related genes when inflammation and immunity are activated, and when external stimulation, such as oxidative stress caused by reactive oxygen species, occurs. Excessive activation has been shown to exacerbate allergic and immune-related diseases including atopic dermatitis and asthma.
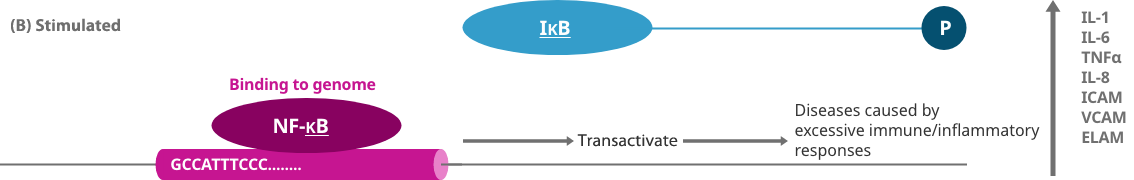
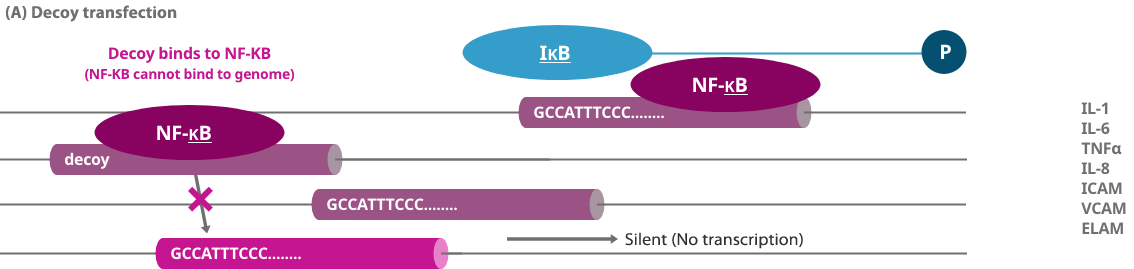
When a gene is expressed, a protein called a transcription factor binds to a specific sequence region of the genome to turn on a switch that controls the production of a protein. Decoy oligonucleotide (NF-κB decoy) is obtained by artificially synthesizing a short nucleic acid (DNA) that contains the same sequence as the transcription factor binding site on the genome. Decoy is a term that originally means a "lure," and a decoy oligonucleotide binds to a specific transcription factor in cells as a lure of a genome, thereby preventing a specific transcription factor from binding to the genome and inhibiting the expression of the gene.
Mechanism of induction of inflammation
When a gene is expressed, a protein called a transcription factor (NF-κB) binds to a specific sequence region of the genome (genome that causes inflammation) and turns on the switch controlling the production of a protein that causes inflammation such as pain.
Decoy for NF-κB, NF-κB Decoy
When an NF-κB decoy is artificially designed as a "lure" and a large number are introduced into the body, the transcription factor NF-κB and a genome that causes inflammation are not bound excessively. In this way, the switch of the genome that causes inflammation becomes difficult to press, and the expression of genes that cause pain is suppressed, thereby reducing pain.
Vaccine
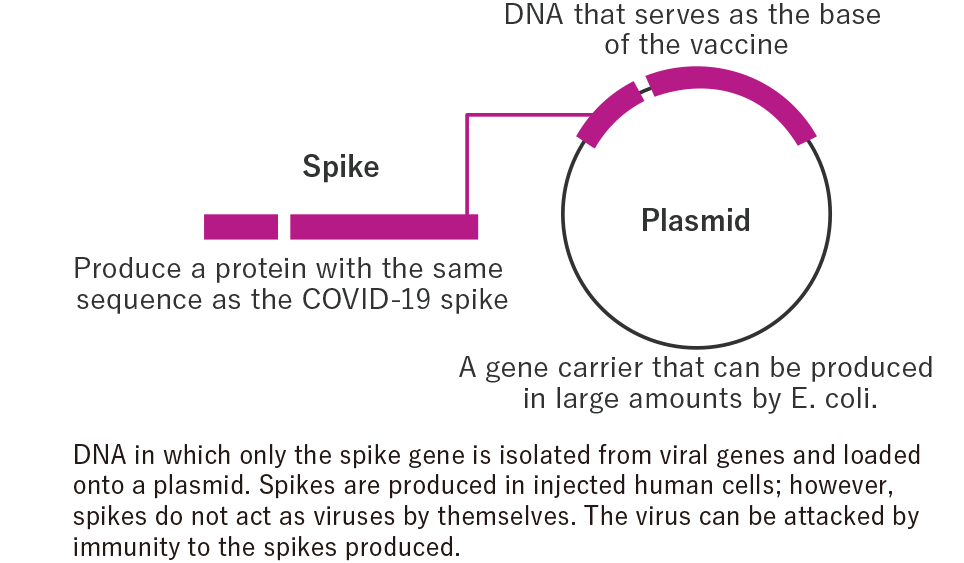
The DNA vaccine is intended to produce a partial protein of pathogen in the body through inoculation of the circular DNA (plasmid) that encodes the protein of the target pathogen and to produce the protein (antigen) and accordingly trigger the antibody-mediated immune response against the pathogen. An antibody that attacks the target antigen (a foreign substance recognized by the body’s immune system) is generated in the body, thereby producing resistance against the antigen and exerting its effect. We are working on the research and development of DNA vaccines utilizing the technologies we have developed for HGF gene therapy products using plasmid DNA.
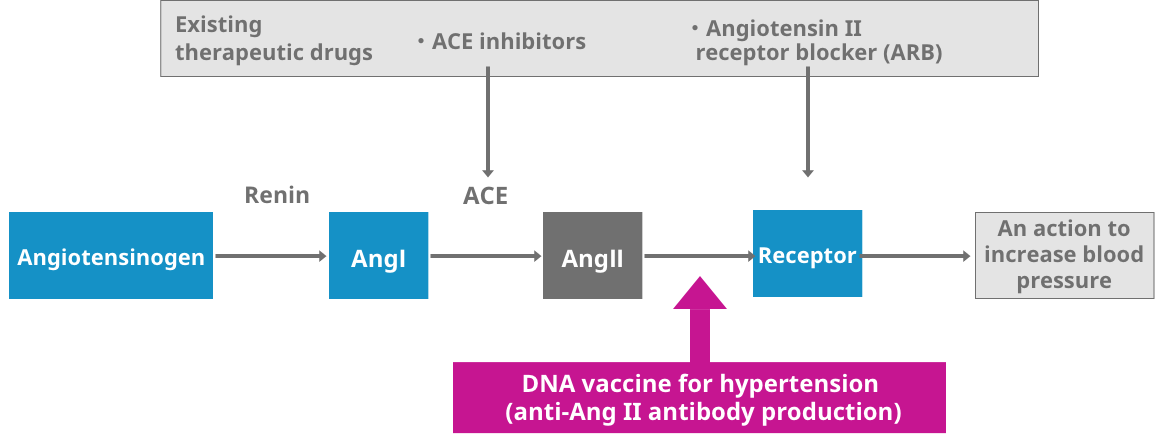
[DNA vaccine for hypertension]
A DNA vaccine for the treatment of hypertension is being developed by producing an antibody that suppresses the action of "angiotensin II," a peptide hormone, the overall effect of which is to increase blood pressure.
It induces the production of an antibody against angiotensin II, a physiologically active substance which increases blood pressure. By attenuating the action of angiotensin II it , exert a stable antihypertensive effect over a prolonged period.
・ It is also under development as a veterinary drug for chronic heart failure in dogs.
(On October 5, 2015, we announced the conclusion of a joint development agreement with DS Pharma Animal Health [a subsidiary of Sumitomo Dainippon Pharma Co., Ltd.].)
Currently, many oral drugs are used for the treatment of hypertension. While these drugs need to be taken every day without fail, a single injection of the DNA vaccine is expected to maintain well compliance for therapy by its effect for a long period.
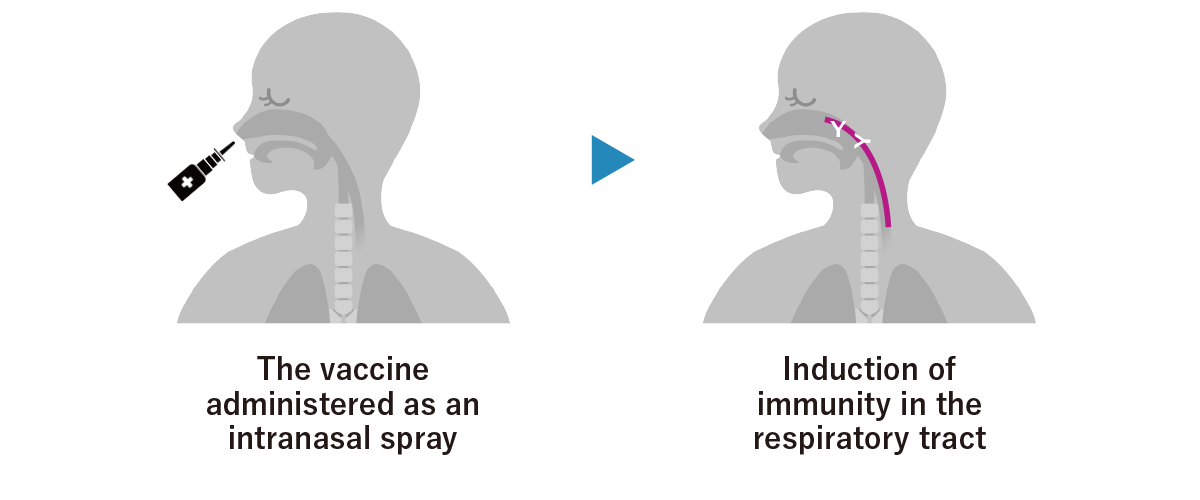
[Intranasal formulation of the COVID-19 vaccine]
Sponsored research and development of an intranasal formulation for a safe and more effective vaccine utilizing Stanford University’s proprietary “Gold-Nanostar Octopod” technology
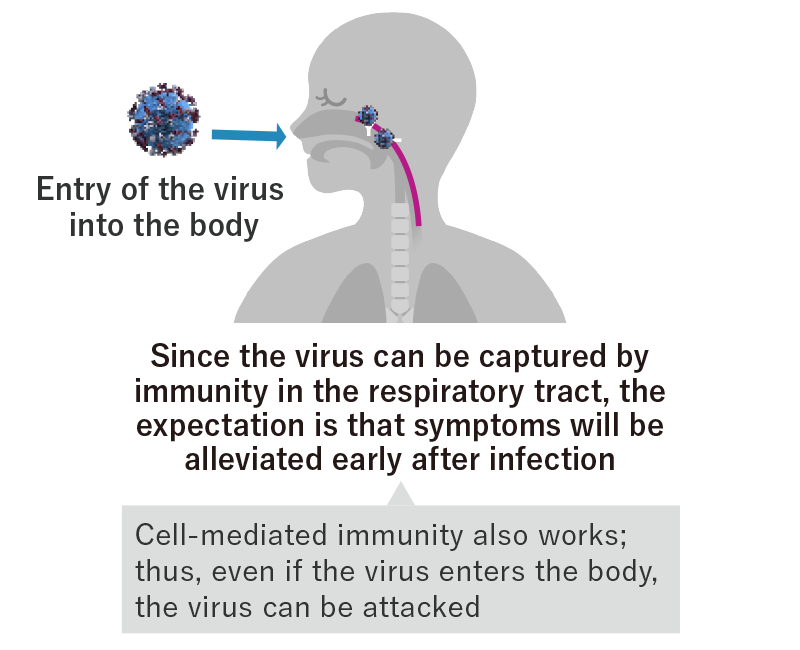
・What is intranasal delivery?
Viruses and bacteria that cause respiratory diseases such as COVID-19, influenza, and the common cold attempt to enter the body through the mucosal membranes of the “upper respiratory tract,” such as the nose, mouth, and throat. The upper respiratory tract has a “mucosal immune system” that attacks viruses to protect the body from infection. The production of “IgA” antibodies, which are a type of antibody secreted into mucosal membranes, in the nose and throat may help to prevent infection itself, and intranasal delivery could create immunity in the part of the respiratory tract that is the site of infection.
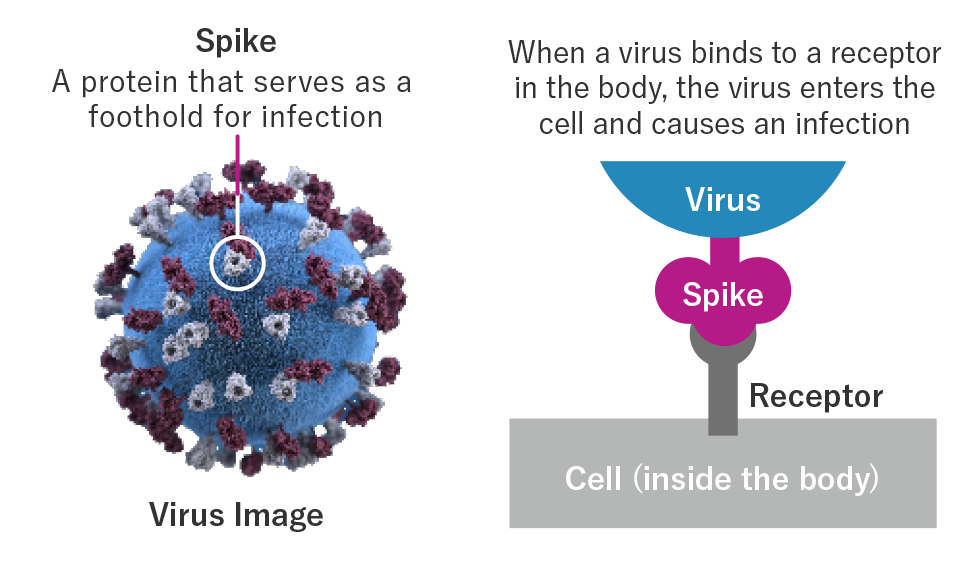
What are viruses?
What are DNA vaccines?
[Other activities]
・CIN therapeutic vaccine
The vaccines for preventing cervical cancer currently on the market in a number of countries are injectable agents intended to prevent infection with human papillomavirus (HPV), and they are not designed to treat cancer in women previously infected with HPV. On the other hand, the vaccine our company is currently developing for the treatment of cervical intraepithelial neoplasia (CIN) is an innovative oral therapy that can eliminate precancerous cervical tissue (high-grade dysplasia) and can be expected to prevent progression to cervical cancer. The CIN vaccine exerts efficacy through a new mechanism by which cell-mediated immunity specific to HPV antigen is activated utilizing intestinal immunity, HPV-infected cells in the cervix are attacked selectively and efficiently, and cancerous cells are eliminated. Our company obtained an exclusive license from BioLeaders Corporation in South Korea to develop, manufacture, use, and market the CIN therapeutic vaccine in Japan, the United States, the United Kingdom, and China. We have entered into a contract to exclusively license this product to Morishita Jintan Co. Ltd.
