about AnGes
Aiming to be a global leader in genetic medicine
In December 1999, AnGes was founded with the aim of developing drugs based on the action of the hepatocyte growth factor (HGF) gene to regenerate blood vessels. The name AnGes was inspired by the English name Angiogenesis, meaning the creation of new blood vessels, and by the desire to be an Ange (French for angel) that delivers new and unprecedented drugs for intractable diseases. This desire has always been held by both the founder and employees.
The concept of treatment called angiogenesis was "unprecedented" at the time. In March 2019, after 20 years of challenges and hard work, AnGes received conditional and time-limited approval as the world's first genetic medicine product using plasmid DNA technology to regenerate blood vessels.
Meanwhile, based on the Global Vascular Guideline published by the Society of Vascular Surgeons of the United States, Europe, and Asia/Oceania in June 2019, we conducted a late stage Phase II clinical trial in mild to moderate arteriosclerosis obliterans with leg ulcers in the United States, and obtained favorable results.
Based on these results, we aim to file for approval in Japan for the treatment of ulcers in a wide range of patients with arteriosclerosis obliterans, regardless of the severity of the disease.
The probability of success in new drug development is said to be one in 30,000, but the fact that we were the first domestic bio-venture to bring an autologous product to market gives us great confidence and further encouragement. At the same time, I feel that it is an important mission to deliver this drug to patients suffering from ischemic diseases around the world, not only in Japan but also overseas.
In Japan, drug loss and drug lag have recently become an issue. While new drugs and treatment methods are being developed one after another in the world, there are many diseases for which there is no treatment available in Japan. As one response to this problem, we acquired the exclusive rights to market Zokinvy, a treatment for Progeria, in Japan, and began sales in May 2024.
In addition, at ACRL, which opened in 2021, We are engaged in screening tests to investigate the possibility of rare genetic diseases for which early detection and treatment are important. With a testing system based on global-standard target diseases, facilities, and technologies, we are increasing the number of orders every year.
In addition, ACRL began contract genetic testing in conjunction with the launch of Zokinvy, and is also engaged in biomarker testing.
At the forefront of research and development as a new treatment method is the technology of genome editing. We entered the development of genome editing by making EmendoBio a subsidiary in 2020. Genome editing is being developed mainly in the U.S. and has begun to be put to practical use as a treatment method for diseases for which there was no cure until now.
EmendoBio's proprietary OMNI nuclease technology is also being used in the development of cutting-edge cancer therapies.
We are committed to the development of genetic medicine with a global perspective and the introduction of therapeutic drugs in Japan, and to meeting the expectations of our stakeholders, including patients and their families who are waiting for new therapeutic drugs and methods of treatment, by developing genome editing technology, identifying diseases before they develop, and providing treatment opportunities. In addition to meeting the expectations of our stakeholders, including patients and their families who are waiting for new treatments and therapies, we will continue to contribute to the realization of healthy lifestyles.
Finally, we would like to thank you for your continued patronage and look forward to your continued support.
Ei Yamada, President & CEO
Values
Contribute to the improvement of human health and quality of life through the development of innovative medicines, by harnessing the potential of genes, acquired over the long course of the humankind.
- AAspiration
- NNetwork
- GGenesis
- EEthics
- SSpeed
Achieve the Corporate Mission by acting along the principles incorporated in the company name “A-N-G-E-S”:
- Aspiration
- Recognize and value the importance of the networks consisting of patients, medical professionals, shareholders, alliance partners, employees and local communities.
- Network
- AnGes believes that every network is a key to creating new value. Patients, shareholders, alliance partners, employees and local communities are vital partners forming the foundation of its networks.
- Genesis
- Continue to incorporate latest research results and ideas, and strive to create new technologies, products and value.
- Ethics
- Work with the highest ethical standards.
- Speed
- Realize innovative gene medicines to ensure prompt delivery to patients in need.
Mission
As a biotech venture company, we focus on research and development of the next-generation biopharmaceuticals such as genetic medicines, and aim to achieve practical use of innovative drugs.
“Genetic medicine” is a new type of biopharmaceutical that utilizes the gene functions. Genetic medicines include gene therapy that is expected to provide innovative effects that can not be obtained with existing treatments, and “nucleic-acid medicines” that perform with high specificity on treatment targets such as decoyoligonucleotides and RNAi.
We are also undertaking the development of therapeutic vaccines, the next-generation biotechnology utilizing DNA plasmid.
It is our mission to make a contribution to the improvement of people’s Quality of Life (QOL) and medical standards through the development of innovative drugs. This is accomplished by utilizing the advanced technology of genetic medicines and therapeutic vaccines for diseases that are intractable or rare, and has no treatments available. We are dedicated to providing innovative and internationally applicable drugs for patients as promptly as possible.
Business Area
- Pipeline
- Gene medicines (e.g. gene therapy and nucleic acid medicine) that use the power of genes to treat intractable and rare diseases and diseases for which there are no effective treatments including development of DNA vaccine
- HGF
gene therapy
product - NF-κB
Decoy Oligonucleotide
DNA - Hypertension
DNA vaccine - COVID-19
DNA Vaccine
- Alliance
- New possible development topics to expand our pipelines for our growth
- Genome editing
- Microbiome
Business Model
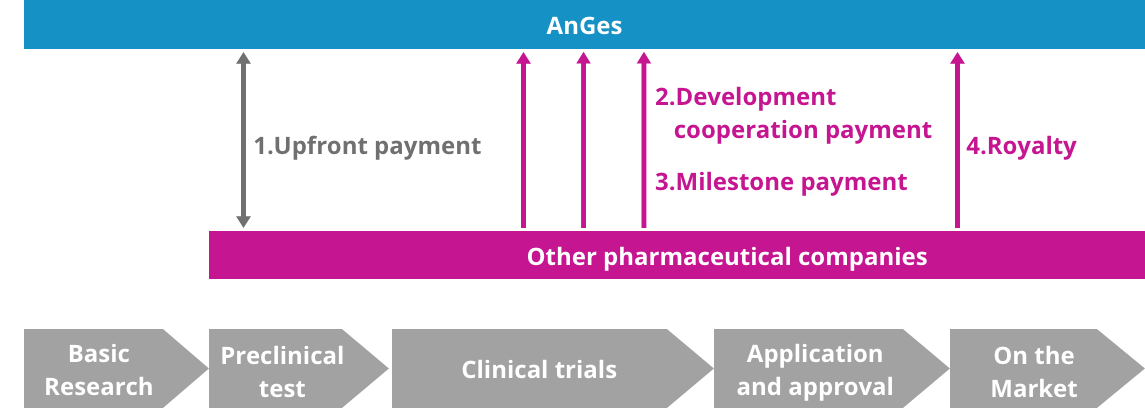
Our Revenue Structure ①Upfront payment: conclusion of agreement ②Development cooperation payment: financial help for R&D ③Milestone payment: R&D progress at agreed stages ④Royalty: percentage of sales post product launch
Company Profile
- Corporate Name
- AnGes , Inc.
- Head Office
- Saito Bio-Incubator, 7-7-15, Saito-asagi, Ibaraki, Osaka, 567-0085 Japan
- Established
- December 17, 1999
- President & CEO
- Ei Yamada
- Capitalization
- 37,255 million yen (As of December 31, 2024)
- Number of Employees
- 55 (As of December 31, 2024 : Consolidated)
- Scope of Business
- Research and Development of Gene-based Medicine
Contract testing for rare genetic diseases
【 Our Company Name 】
Company name "AnGes" comes from the French word "Ange" meaning angel.
The word "AnGes" includes "Angiogenesis," and "Anti-gene," meaning gene regulation.
【 Corporate Brochure 】
Board of Directors
President and CEO, Ei Yamada
- April 1981
- Special Researcher, Japan Society for the Promotion of Science
- April 1982
- Mitsubishi Kasei Corporation (currently Mitsubishi Chemical Corporation)
- January 1995
- Sosei K.K.
- August 2000
- Takara Shuzo Co., Ltd.
Director, Dragon Genomics Inc. (currently Takara Bio Inc.) - May 2001
- General Manager of Business Development, AnGes, Inc.
- August 2001
- Member of the Board, AnGes, Inc.
- June 2002
- CEO, AnGes Euro Limited
- September 2002
- President and CEO, AnGes, Inc. (current)
- March 2014
- CEO, AnGes USA, Inc.
- January 2020
- External Board Member, Barcode Diagnostics Ltd. (current)
Member of the Board, EmendoBio Inc. - September 2023
- Member of the Board, Emendo Research and Development Ltd. (current)
- March 2024
- CEO, EmendoBio Inc. (current)
Member of the Board Naoya Sato
- April 1985
- Joined Mitsubishi Kasei Corporation(currently Mitsubishi Chemical Corporation)
- April 2010
- Manager, International Business Department, Mitsubishi Tanabe Pharma Corporation
- April 2013
- General Manager, Department Ⅰ, Pharmacology Research Laboratories Ⅱ, Mitsubishi Tanabe Pharma Corporation
- June 2015
- Seconded as Specially Appointed Professor, TMK Project, Medical Innovation Center, Graduate School of Medicine, Kyoto University
- May 2020
- Joined AnGes, Inc.
Director of Office of the President - October 2021
- Director of Corporate Development, AnGes, Inc.(current)
- March 2022
- Member of the Board and Director of Corporate Development, AnGes, Inc. (current)
- September 2022
- Member of the Board, EmendoBio Inc. (current)
- June 2023
- External Board Member, MyBiotics Pharma Ltd. (current)
- September 2023
- Member of the Board, Emendo Research and Development Ltd.
- March 2024
- CEO, Emendo Research and Development Ltd. (current)
Member of the Board Norikazu Eiki
- August 1979
- Nihon Ciba-Geigy K.K.
- January 1994
- Bayer Yakuhin, Ltd
- March 1997
- Director (Shiga Factory Manager), Bayer Yakuhin, Ltd.
- July 2002
- Representative Director & President, Bayer Yakuhin, Ltd.
- January 2007
- Representative Director & Chairman, Bayer Yakuhin, Ltd.
- April 2010
- Director & Chairman, Bayer Yakuhin, Ltd.
- May 2014
- Member of the Board(External Director), AnGes, Inc. (current)
- June 2015
- Outside Director, TOWA PHARMACEUTICAL CO., LTD. (current)
- April 2016
- External Director, Solasia Pharma K.K. (current)
- January 2017
- Outside Director, FunPep Co., Ltd. (current)
- June 2018
- External Board Director, Gene Techno Science Co.,Ltd. (current)
Member of the Board Makoto Hara
- April 1974
- Sumitomo Chemical Co., Ltd. (currently Sumitomo Chemical Company Limited)
- August 1999
- General Manager, Corporate Planning Office, Sumitomo Pharmaceuticals Co., Ltd.
General Manager, Pharmaceutical Operations Office, Sumitomo Chemical Company Limited - April 2003
- General Manager, Petrochemicals & Plastic Office, Sumitomo Chemical Company Limited
- June 2005
- Executive Officer, General Manager, Corporate Planning & Coordination Office, Finance & Accounting, Sumitomo Chemical Company Limited
- April 2008
- Managing Executive Officer, Sumitomo Chemical Company Limited
- April 2010
- Senior Managing Executive Officer, Sumitomo Chemical Company Limited
- September 2010
- Senior Executive Officer, Sumitomo Dainippon Pharma Co., Ltd.
- June 2011
- Member, Board of Directors, Senior Executive Officer, Sumitomo Dainippon Pharma Co., Ltd.
- April 2012
- Member, Board of Directors, Executive Vice President, Sumitomo Dainippon Pharma Co., Ltd.
- June 2016
- Advisor, Sumitomo Dainippon Pharma Co., Ltd.
- March 2018
- Member of the Board, AnGes, Inc. (current)
- March 2024
- Outside Director, FunPep Co., Ltd. (current)
Member of the Board Yasue Mitsukura
- April 1999
- Research assistant, Tokushima University
- December 2001
- Full-time lecturer, Okayama University
- January 2005
- Associate professor, Tokyo University of Agriculture and Technology
- April 2005
- Visiting Professor, The University of Tokyo (concurrent)
- April 2011
- Associate professor, Keio University
- August 2013
- Director and CTO, Dentsu ScienceJam Inc. (current)
- April 2018
- Professor, Keio University (Faculty of Science and Technology, School of Medicine) (current)
- October 2023
- Representative Director and President, FeMup Co., Ltd. (current)
- October 2023
- Representative Director and President, IKI Inc. (current)
- November 2024
- Representative Director and President, IKI Japan Inc. (current)
- January 2025
- A.ROUND Co., Ltd. (current)
Standing Corporate Auditor Ikuo Mori
- April 1979
- Joined the Ministry of Finance, assigned to the Budget Bureau
- July 1985
- Associate, Salomon Brothers Inc (New York)
- September 1988
- Vice President, Shearson Lehman Hutton Securities Inc.
- September 1989
- Vice President, Shearson Lehman Hutton Inc. (New York)
- September 1991
- Assistant Director, Barclays de Zoete Wedd (London)
- January 1993
- Director, Barclays Securities Japan Limited
- June 1997
- Senior Representative in Japan, CAL FP Bank
- July 1998
- General Manager, RECOF Corporation
- February 2001
- Director, BNP Paribas Securities (Japan) Limited
- October 2006
- Director, KPMG FAS Co., Ltd.
- September 2012
- Managing Director, KPMG FAS Co., Ltd.
- July 2018
- Managing Director, Lincoln International LLC
- January 2023
- Representative, Mori Associates (current)
Corporate Auditor Kiyotaka Hayashi
- April 1979
- Nomura Securities Co., Ltd.
- December 1997
- General Manager, Toyama Branch Office, Nomura Securities Co., Ltd.
- June 2000
- General Manager, Business Development & IPO Department., Nomura Securities Co., Ltd.
- April 2002
- General Manager, Corporate Finance Department, Nomura Securities Co., Ltd.
- April 2009
- Seconded as Director, Nomura Investor Relations Co., Ltd.
- April 2012
- Managing Director, Nomura Investor Relations Co., Ltd.
- April 2015
- Seconded as General Manager, Sales Development Division, Pronexus Inc.
- December 2016
- Transferred to Pronexus Inc.
- April 2018
- Executive Officer, Pronexus Inc.
- April 2020
- Managing Executive Officer, General Manager, Solution Business Division, Pronexus Inc.
- June 2021
- Director and Managing Executive Officer, General Manager, Solution Business Division, Pronexus Inc.
- July 2023
- Joined SBI Holdings, Inc. Seconded as Director, SBI ALApromo Co., Ltd.
- June 2024
- Statutory Auditor, SBI Leasing Services Co., Ltd. (current)
Corporate Auditor Hideyuki Yamanashi
- April 1984
- Ajinomoto Co., Inc.
- April 1997
- General Manager, Accounting & Finance Group, General Affairs Department, Ajinomoto Frozen Foods Co., Inc.
- July 2002
- Director in charge of finance, Ajinomoto Co., (Thailand) Ltd.
- July 2008
- Associate General Manager, Corporate Planning Department, Ajinomoto Co., Inc.
- December 2011
- General Manager, Finance Department, Ajinomoto Pharmaceuticals Co., Ltd. (currently EA Pharma Co., Ltd.)
- July 2013
- General Manager, Corporate Planning Department, Ajinomoto Pharmaceuticals Co., Ltd.
- July 2016
- General Manager, the Corporate Strategy & Management, J-OIL MILLS, Inc.
- September 2017
- Associate General Manager, Audit & Supervisory Board Office, Ajinomoto Co., Inc.
- July 2021
- Audit Committee staff, Planning Group, Internal Auditing Department, Ajinomoto Co., Inc. (current)
Locations
- Head Office
- Saito Bio-Incubator, 7-7-15, Saito-asagi, Ibaraki, Osaka, 567-0085 Japan
- Tokyo Office
- 9F, PMO TAMACHI Ⅱ, 4-13-3, Shiba, Minato-ku, Tokyo, 108-0014 Japan
- Tonomachi R&D Center
(CMC/ Drug Discovery Research) - Innovation Center of NanoMedicine, 3-25-14, Tonomachi, Kawasaki-ku, Kawasaki,
Kanagawa, 210-0821 Japan - AnGes Clinical Research Laboratory (ACRL)
- Life Science & Environment research center, 3-25-13, Tonomachi, Kawasaki-ku, Kawasaki,
Kanagawa, 210-0821 Japan
History
- December 1999
- Founded as MedGene Co., Ltd. in Izumi-city, Osaka for research and development of gene and nucleotide based drugs and reagents for use in functional analyses of genetic medication.
- June 2000
- Changed corporate name to MedGene Bioscience Co., Ltd.
- January 2001
- Established Tokyo branch in Minato-ku, Tokyo.
- October 2001
- Changed corporate name to AnGes MG, Inc.
- October 2001
- Founded AnGes, Inc. in the state of Maryland for clinical development in the US.
- June 2002
- Founded AnGes, Euro Ltd. (business development in Europe) in the county of Sussex, UK.
- July 2002
- Founded GenomIdea Inc. in Toyonaka-city, Osaka for gene function analyses.
- September 2002
- IPO: AnGes MG achieved listing on the Mothers markets of Tokyo Stock Exchange.
- September 2003
- Transferred HVJ envelope vectors business to GenomIdea, Inc.
- March 2004
- Changed corporate name to AnGes MG, Inc.
- September 2004
- Moved head office to Ibaraki-city, Osaka.
- September 2004
- Moved head office of GenomIdea Inc. to Ibaraki-city, Osaka.
- December 2006
- Formed partnership with BioMarin Pharmaceutical, Inc. on marketing and distribution of a product approved in the US, “Naglazyme®” (gulsulfase) for treatment of the genetic disease
- April 2008
- Launched "Naglazyme®" for treatment of mucopolysaccharidosis VI in Japan.
- December 2010
- Formed license agreement with Shionogi & Co., Ltd. on co-development of NF-kB Oligonucleotide for atopic dermatitis
- October 2012
- Formed definitive agreement with Mitsubishi Tanabe Pharma Corporation for exclusive marketing rights of Collategene® in the United States
- January 2013
- Transferred the stock in GenomIdea, Inc. to Ishihara Sangyo Kaisha, Ltd.
- June 2015
- Formed definitive agreement with Mitsubishi Tanabe Pharma Corporation for exclusive marketing rights of Collategene® in Japan
- July 2017
- Changed corporate name to AnGes, Inc.
- January 2018
- HGF Plasmid Marketing approval application submitted
- March 2019
- Obtained conditional time-limited authorization in Japan for HGF gene therapy product Collategene® to treat critical limb ischemia
Transferred marketing rights of Naglazyme®, a treatment for mucopolysaccharidosis type VI to BioMarin Pharmaceutical Japan - September 2019
- Launched Collategene® as HGF gene therapy product for critical limb ischemia in Japan
- March 2020
- Launched co-development of DNA vaccine with Osaka University for novel coronavirus disease (COVID-19)
- December 2020
- Launched co-development of AV-001 with Vasomune Therapeutics, Inc., for the Treatment of hospitalized patients with COVID-19
- December 2020
- Acquired genome editing technology company, EmendoBio Inc. as a subsidiary
- April 2021
- Established AnGes Clinical Research Laboratory
- May 2022
- Eiger BioPharmaceuticals and AnGes Announce Exclusive Partnership for Regulatory Approval and Commercialization of Zokinvy® (lonafarnib) in Japan
- September 2022
- Discontinues development of DNA vaccine for prevention of novel coronavirus infection in collaboration with Osaka University
- May 2023
- Filed for manufacturing and marketing approval for Zokinvi, a product for the treatment of Progeria.
Filed for manufacturing and marketing approval of Collategene, a product for HGF gene therapy. - Oct. 2023
- Administration of NF-κB decoy oligo DNA for the treatment of chronic discogenic lumbago begins in domestic Phase II clinical trials.
- January 2024
- Received manufacturing and marketing approval for Zokinvy for the treatment of Progeria.
- May 2024
- Launched Zokinvy for the treatment of Progeria.
- June 2024
- Withdrawal of application for approval and termination of sales of Collategene, an HGF gene therapy product.
Agreed with Mitsubishi Tanabe Pharma Corporation to terminate the marketing agreement for “Collategene” in Japan and the U.S.
Group
AnGes and two consolidated subsidiaries, are engaged in the development and marketing of gene therapy products.
AnGes USA, Inc.
111 Town Square Place, Suite 1140, Jersey City, New Jersey, 07310 USA
EmendoBio Inc.
111 Town Square Place, Suite 1140, Jersey City, New Jersey, 07310 USA
<Our Business>
・AnGes, Inc.
Development and marketing of Gene Medicine, Gene Therapy Products and DNA Vaccine
・AnGes USA, Inc.
U.S. based development of Gene Medicine
・EmendoBio Inc.
Development of genome editing technology platform and products for gene therapy using genome editing technology
Alliance Status
AnGes aims at becoming a “global innovator in the field of gene medicine” and prioritizes, as its most significant strategies, the acquisition of promising seeds (patents or technologies based on which drugs are discovered and developed) that are suited for its product development plan and the alliance for drug development. As of today, several promising seeds have been obtained from universities in Japan and the following alliances have been formed.
AnGes has a technology franchise in the cutting-edge field of gene medicine including gene therapy and nucleic acid medicine and has been leading the world in research and development in this field. As a result, AnGes has accumulated a wide variety of know-how in research and development as well as in technology in the field of gene medicine. Using such intellectual property, AnGes will continue to develop innovative drugs and explore new technologies in the future.
<Licensee of sales rights>
| Project | Indication | Area | Partner | |
|---|---|---|---|---|
| HGF Plasmid (Beperminogene perplasmid) |
Peripheral Arterial Diseases (Arteriosclerosis Obliterans & Buerger's Disease) |
Israel |  |
Kamada |
| Turkey |  |
Er-Kim | ||
<In-Licensed Project>
| Project | Indication | Our Rights | Partner |
|---|---|---|---|
| CIN Therapeutic Vaccine | Cervical Intraepithelial Neoplasia | development and marketing rights for Japan, US, England and China | BioLeaders (South Korea) Morishita Jintan, Co. (JP) |
| Lonafarnib | Premature aging diseases (HGPS・PL)* | the regulatory approval, marketing, and distribution in Japan | Sentynl Therapeutics, Inc. (US) |
*「HGPS」: Hutchinson-Gilford progeria syndrome / 「PL」: Progeroid laminopathies
<Group>
| Project | Relationship | Company | |
|---|---|---|---|
| Development of genome editing technology platform and products for gene therapy using genome editing technology | Consolidated subsidiaries | EmendoBio Inc. (US) | |
<Strategic Alliance>
| Project | Alliance | Partner | |
|---|---|---|---|
| Microbiome-cultivation and formulation of indigenous bacteria | Co-development |  |
MyBiotics Pharma (Israel) |
| COVID-19/ARDS | Co-development |  |
Vasomune Therapeutics (Canada) |
| COVID-19 DNA Vaccine | Co-development | Stanford University | |

