ACRLAnGes Clinical Research Laboratory
What is AnGes Clinical Research Laboratory?
AnGes Clinical Research Laboratory (ACRL), which opened in April 2021, is an inspection laboratory*1
the main purpose of which is to perform testing for rare genetic diseases.

AnGes's efforts to address intractable and rare diseases
Since its founding, AnGes has been researching and developing drugs for rare and intractable diseases.
Although more rare genetic diseases have become treatable, there are certain diseases for which, even if they are treatable, the desired therapeutic effect can be achieved only when treatment is initiated before symptom onset. Some other diseases have few characteristic symptoms and are difficult to detect with standard diagnostic techniques. It is important that the treatment of rare genetic diseases should be initiated early in the course of disease, preferably before the onset of disease.
For rare genetic diseases, newborn mass screening, which tests all newborns born in Japan for inherited metabolic diseases*2, is currently provided free of charge as a public program, mainly by local governments in each region, leading to early detection and treatment.
For diseases caused by genetic changes that can be detected and treated at an early stage other than those covered by the newborn mass screening program, local governments and organizations in each region offer fee-based additional testing for those who wish to receive it.
Since April 2021, our ACRL has been contracted to provide testing services for optional screening*3, fee-based testing provided by the Clinical & Research Association for Rare, Intractable Diseases (CReARID), for those who wish to receive testing.
In the future, we will also seek to be contracted to provide additional testing services for diseases (rare genetic diseases) not covered by the newborn mass screening, which are being performed by local governments, organizations, and laboratory companies nationwide, thereby contributing to the promotion of awareness and understanding of rare genetic diseases and the dissemination of treatment for such diseases.
- *1)It is a facility that inspects inspection materials collected or excreted from the human body. This facility is registered based on the Act on Medical Technologist.
- *2)Inborn errors of metabolism are diseases in which metabolism is impaired due to the natural inability of enzymes and transporters to function properly, and abnormal substances are accumulated in the body or the body lacks necessary substances, resulting in a variety of symptoms.
- *3)For more information on optional screening, please visit CReARID's website.
Additional testing for rare genetic diseases
Additional testing for rare genetic diseases is to determine the likelihood of having a disease caused by genetic changes. It is testing for diseases that meet the following criteria:
- Diseases for which treatments are available and early detection and treatment is highly beneficial.
- Diseases that are difficult to diagnose due to their rarity and for which sufficient therapeutic effect cannot be obtained when treatment is initiated after symptoms have progressed due to delayed diagnosis, whereas detection before onset can enhance the effectiveness of treatment, prevent the onset of the disease, and avert serious disability.
Currently, ACRL is performing additional testing for nine rare genetic diseases: mucopolysaccharidosis types I, II, IVA, and VI, Fabry disease (male infants only), Pompe's disease, adrenoleukodystrophy (male infants only), spinal muscular atrophy (SMA), and severe combined immunodeficiency. We will gradually expand the scope of testing to cover other diseases.
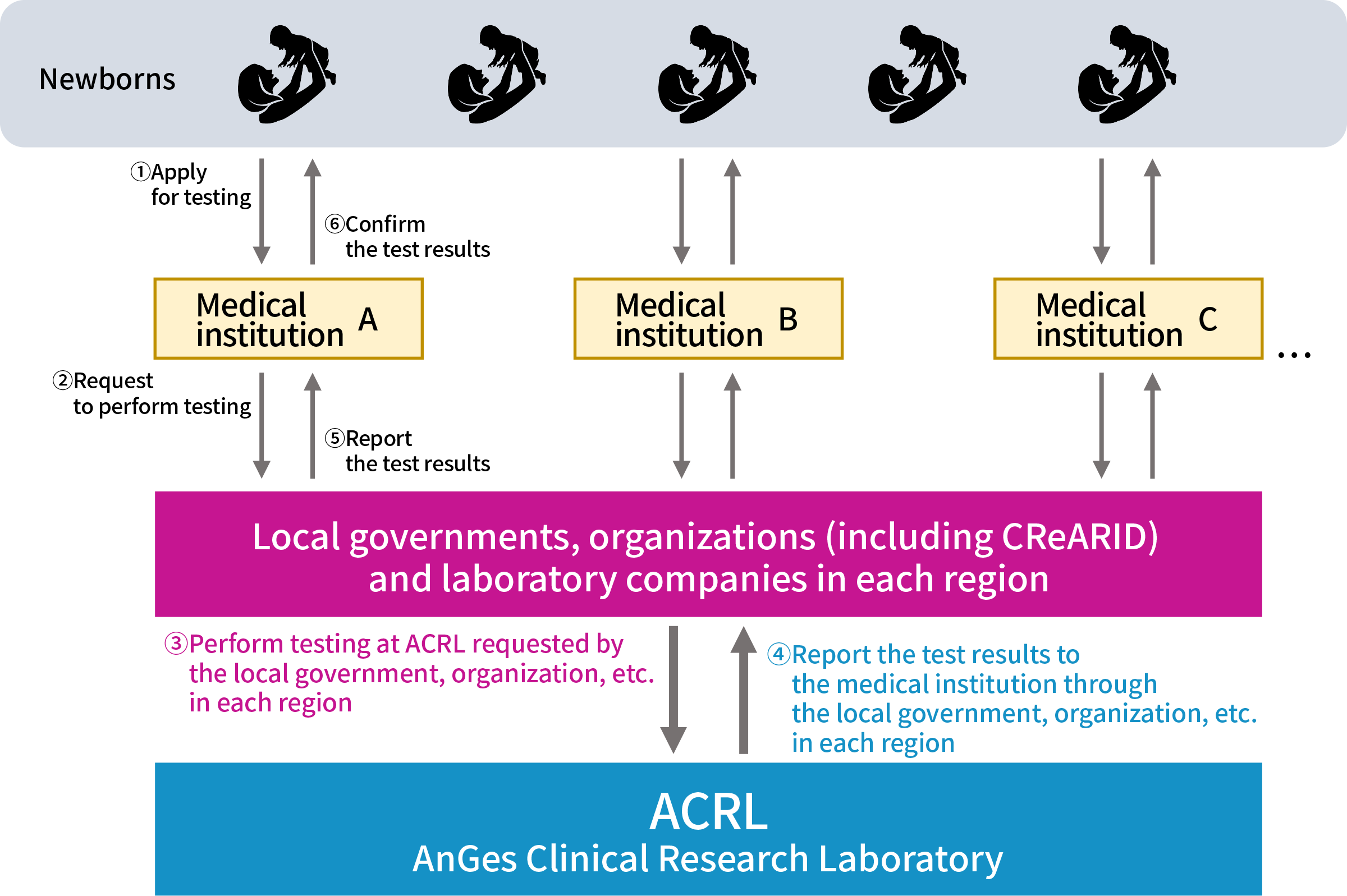
Flow of additional testing for rare genetic diseases
- ① Parents of newborns and infants: Apply for testing for inherited metabolic diseases at a medical institution
- ② Medical institutions: Request local governments, organizations, laboratory companies, etc. in each region to perform testing for rare genetic diseases
- ③ Municipalities, organizations, laboratory companies, etc.: Request ACRL to perform testing for rare genetic diseases
- ④ ACRL: Perform testing and report the test results to the local government, organization, or laboratory company in each region
- ⑤ Municipalities, organizations, laboratory companies, etc.: Report the test results to the medical institution
- ⑥ Parents of newborns and infants: Confirm the test results at the medical institution where they applied for the testing
Q & A
What is additional testing for rare genetic diseases?
It is fee-based testing to determine the likelihood that newborns have diseases caused by genetic changes (rare genetic diseases) that are not covered by the newborn mass screening provided free of charge.
How can I request additional testing for rare genetic diseases?
Please contact the corresponding medical institution in your region.
As a rule, a small amount of blood required for the test is additionally drawn at the time of blood collection for newborn mass screening, thus minimizing the burden on the baby.
Which rare genetic diseases are covered by the additional testing?
The additional testing covers the following nine diseases: mucopolysaccharidosis types I, II, IVA, VI, Fabry's disease (male infants only), Pompe's disease, adrenoleukodystrophy (male infants only), spinal muscular atrophy (SMA), and severe combined immunodeficiency.
How soon can I obtain the results of additional testing for rare genetic diseases?
You can obtain the results at the medical institution you visited at the one-month checkup.
What will happen if the test result is positive?
Please consult the medical institution you visited.
